*Please scroll down to the bottom of the page for the final deliverable.
Project Overview
Dimerix is a clinical stage biotechnology company focused on developing new therapeutics discovered using their proprietary drug development Receptor-Heteromer Investigation Technology platform. Dimerix’s lead therapy DMX-200 is currently in Phase II clinical trials for chronic kidney disease and has received US Orphan Drug Designation for Focal Segmental Glomerulosclerosis.
The primary objective of this project is to attract the interest of investors. The secondary objective is to create new business partnerships. Video 2 explains the Receptor-Heteromer Investigation Technology.
The target audience for video 2 are scientifically trained and have a reasonably good understanding of the drug discovery process.
Storyboard
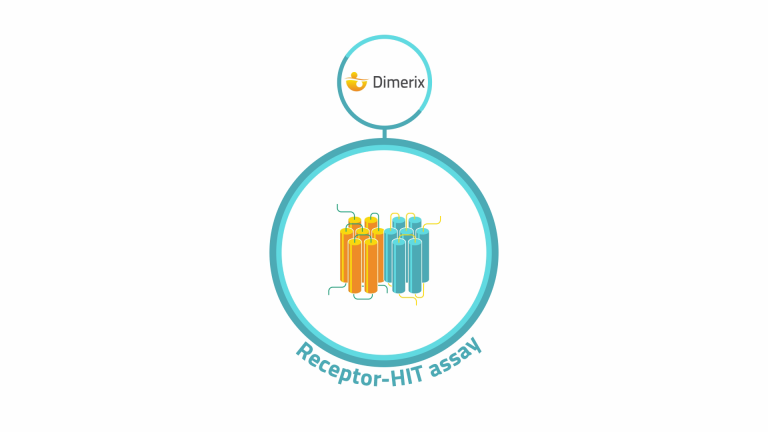
At Dimerix, we’ve developed our cell-based Receptor-HIT assay to identify and interrogate GPCR heteromers.
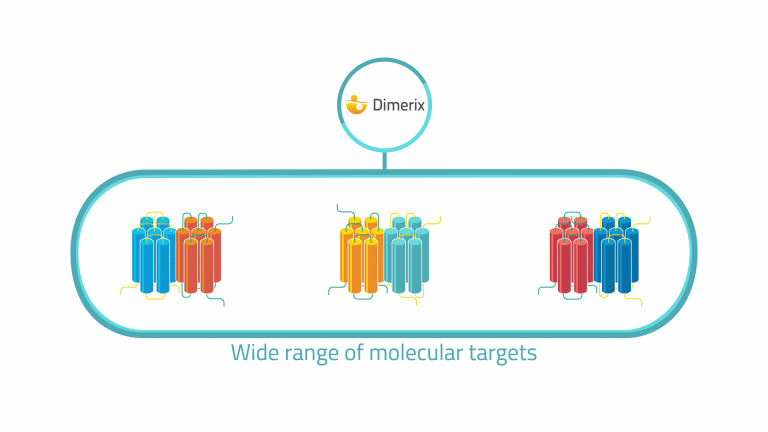
This flexible platform works with a wide range of molecular targets, reducing the risk of unidentified signaling interactions.
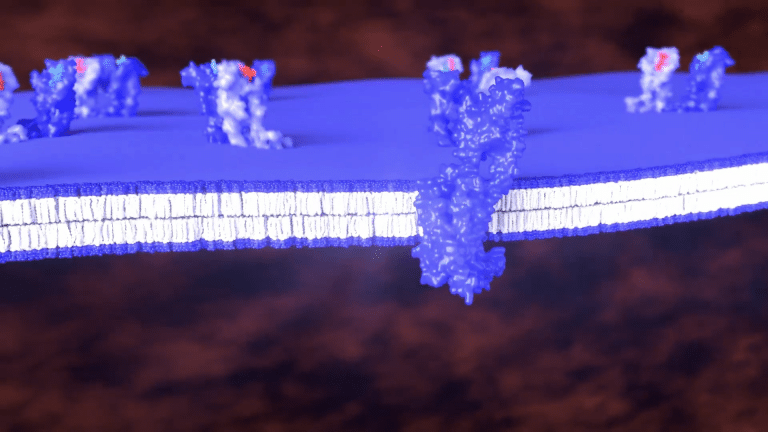
Our patented BRET assay can be configured as a three-component system comprised of an unlabeled GPCR,
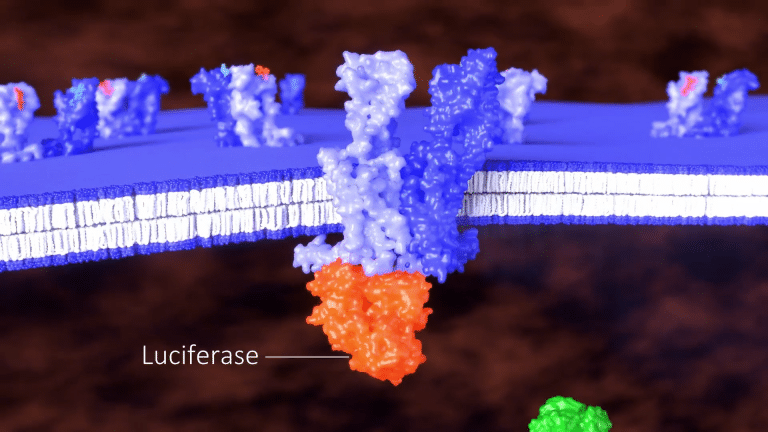
a second GPCR fused to luciferase,
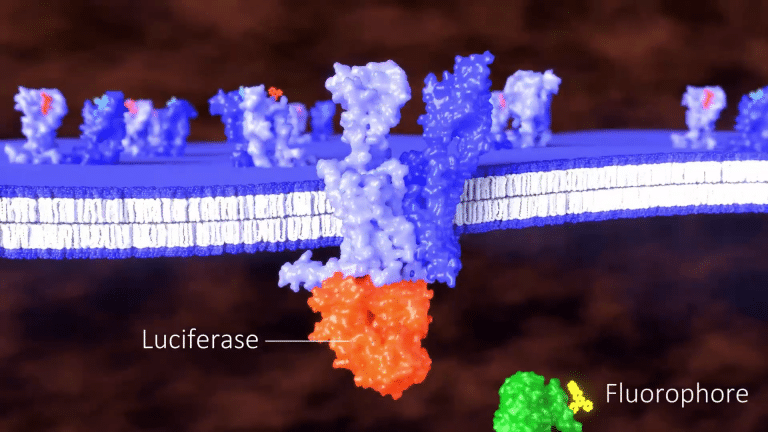
and a signaling molecule fused to a fluorophore.
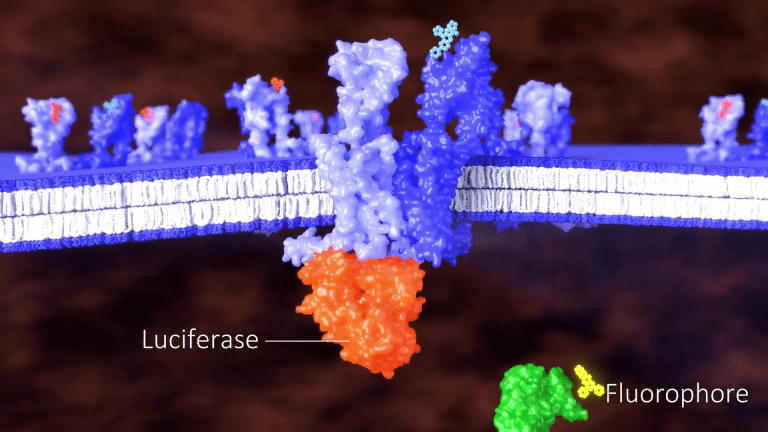
When a ligand binds to the unlabeled GPCR and heteromerizes with the labeled GPCR,
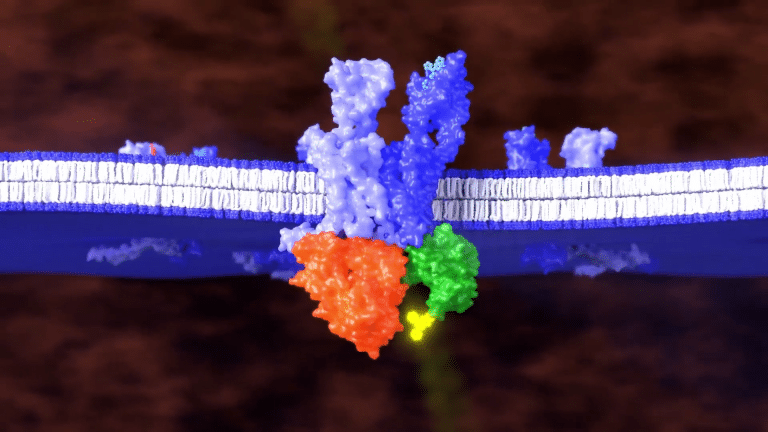
the fluorophore is brought close enough to the luciferase to generate a signal.
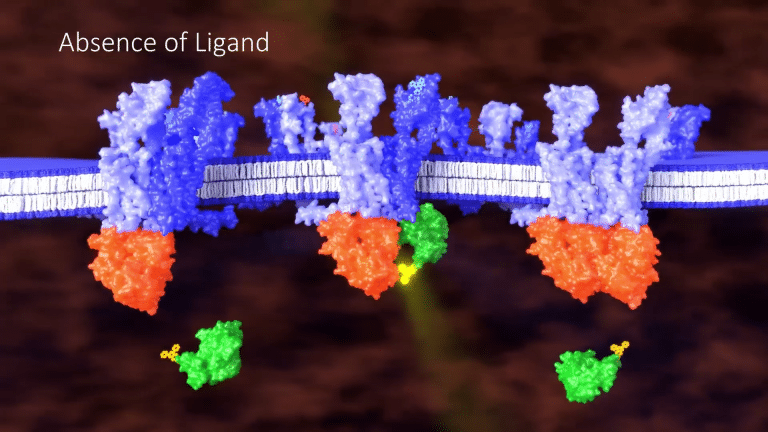
Because this assay is highly dependent on proximity, no signal is generated in the absence of a ligand
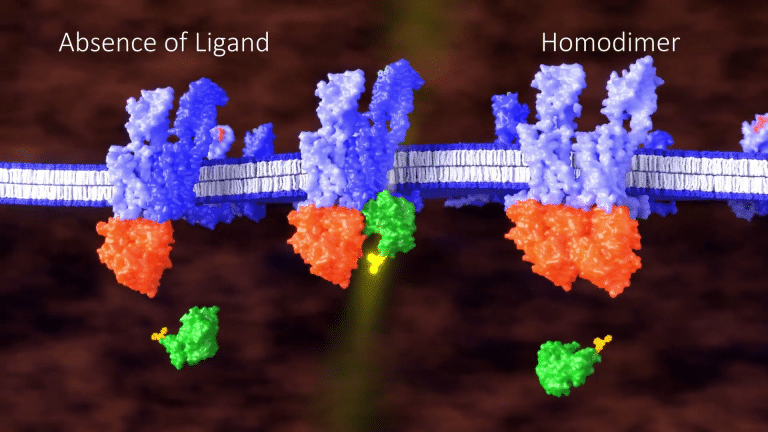
or when the GPCRs form homodimers, resulting in a robust assay with low background.
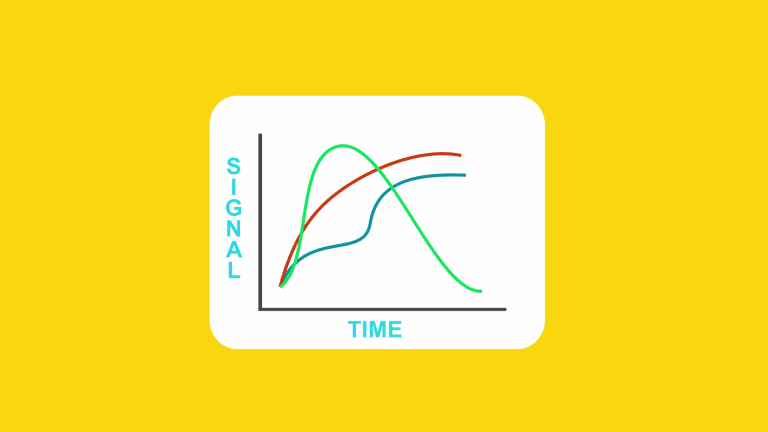
Due to its unique ability interrogate the pharmacodynamics of GPCR complexes,
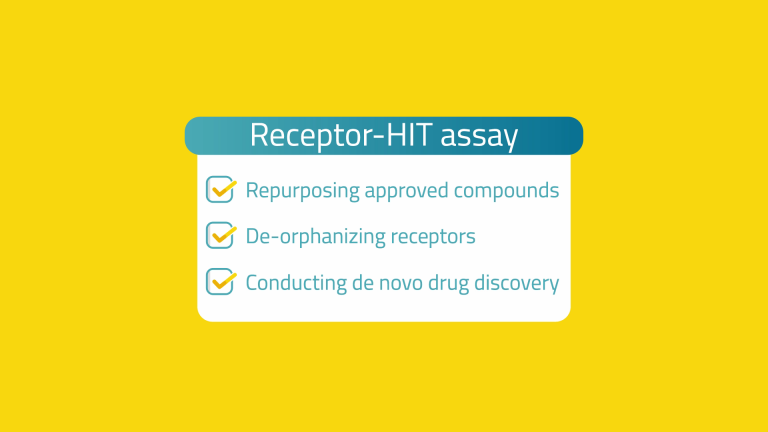
our Receptor-HIT assay is great for repurposing approved compounds, de-orphanizing receptors, and conducting de novo drug discovery.

To see how you can partner with Dimerix and take advantage of this powerful technology, email info@dimerix.com.