*Please scroll down to the bottom of the page for the final deliverable.
Project Overview
Dimerix is a clinical stage biotechnology company focused on developing new therapeutics discovered using their proprietary drug development Receptor-Heteromer Investigation Technology platform. Dimerix’s lead therapy DMX-200 is currently in Phase II clinical trials for chronic kidney disease and has received US Orphan Drug Designation for Focal Segmental Glomerulosclerosis.
The primary objective of this project is to attract the interest of investors. The secondary objective is to create new business partnerships. This is done through a primary video (video 1) which explains the mechanism of action of Dimerix’s lead therapy.
The target audience for video 1 is non-scientific lay persons. They may have a general understanding of some of the scientific concepts surrounding kidney disease and are likely to be quite an intelligent audience. Nonetheless the video needs to be pitched at non-scientists.
Storyboard
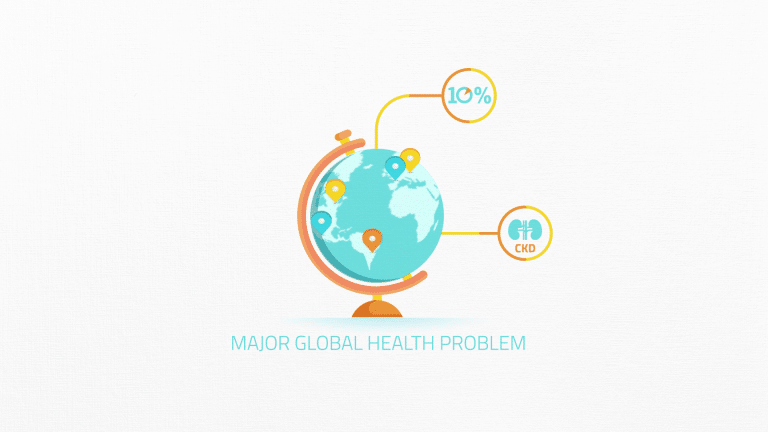
Affecting over ten percent of the population, chronic kidney disease, or CKD, is a major global health problem.
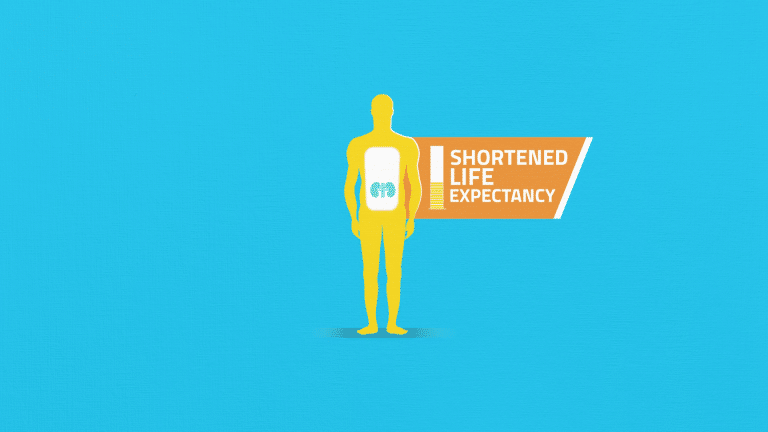
Sufferers experience reduced quality of life and a shortened life expectancy, and many ultimately require dialysis.
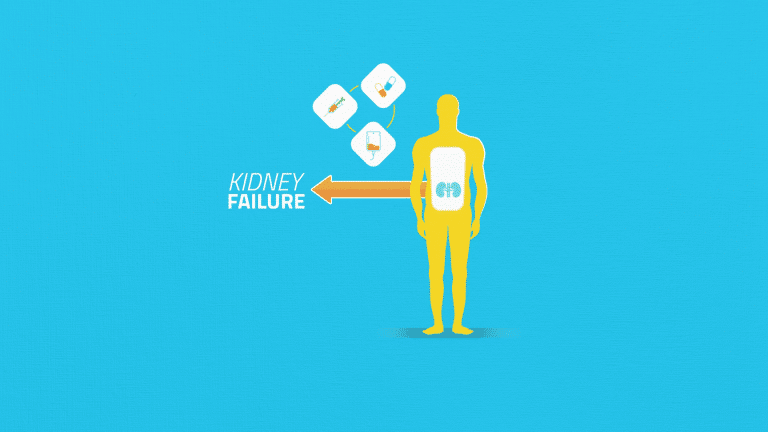
Current therapeutic approaches only slow disease progression towards kidney failure and new approaches are needed,

which is why Dimerix is developing DMX-200, an innovative new therapy in clinical trials.

What is CKD?
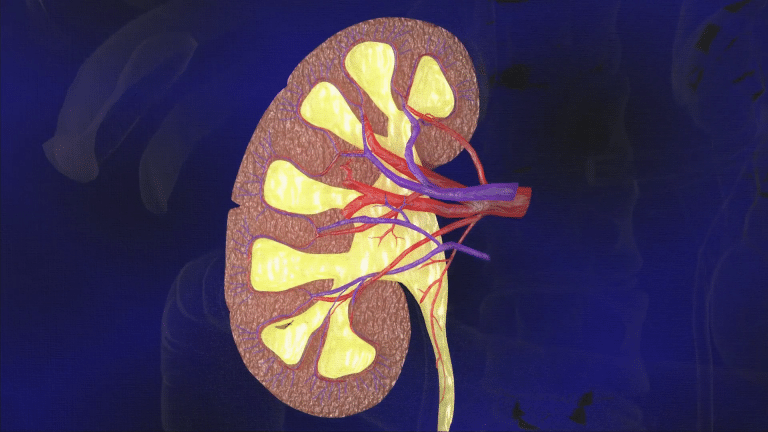
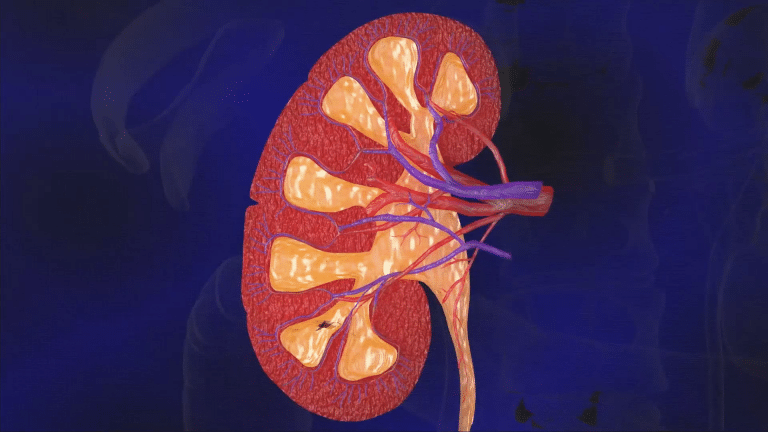
CKD includes a number of different conditions where the kidneys are damaged
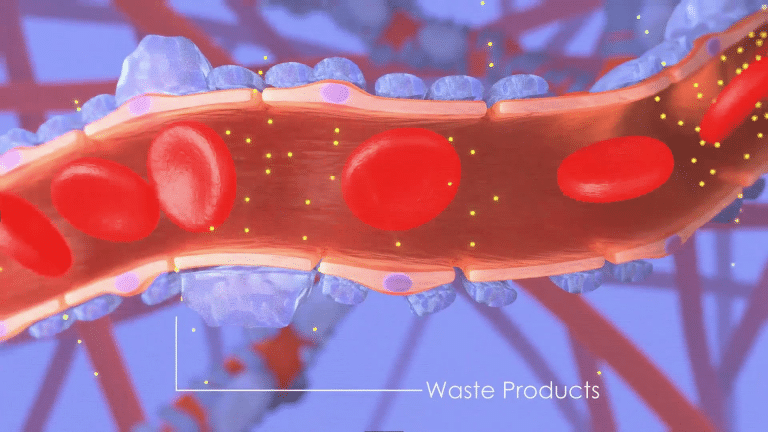
and no longer able to efficiently filter waste out of the blood.
For many CKD patients, damage stems from an abnormal inflammation response where the immune system inappropriately attacks kidney cells.
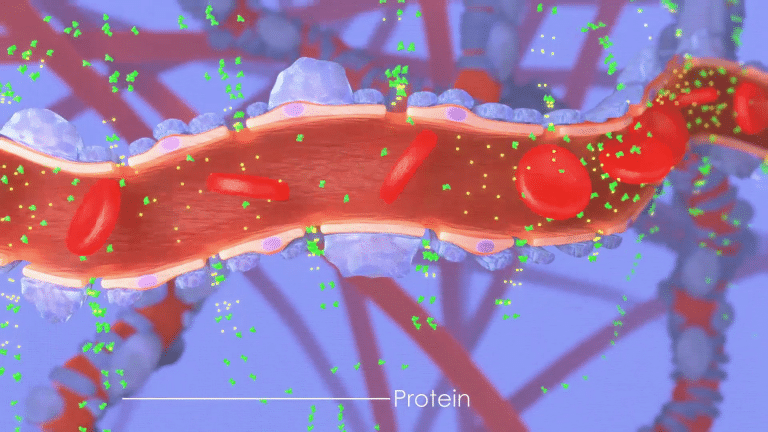
This attack compromises the kidney’s ability to filter the blood, permitting leakage of abnormally high levels of protein from the blood into the urine,
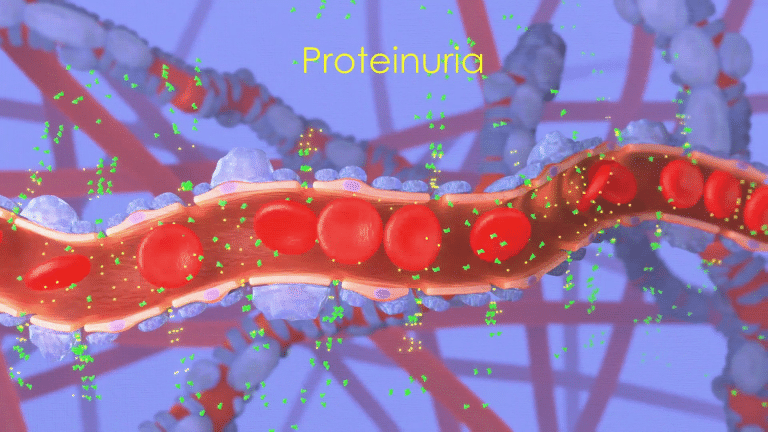
a condition called proteinuria.
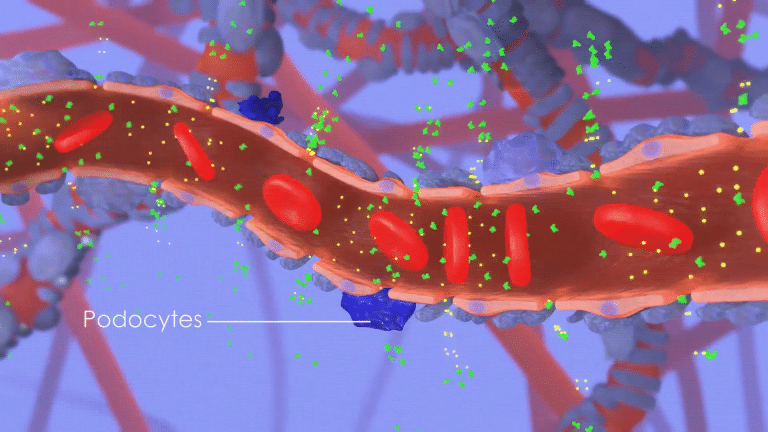
Unfortunately, proteinuria itself is also believed to cause kidney damage,
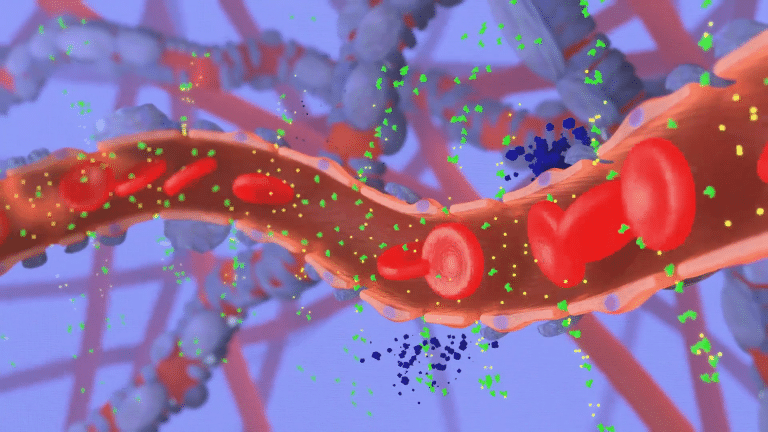
compounding cellular injury and disease progression.

The current standard of care for patients with CKD includes blood pressure-lowering treatments such as irbesartan, a drug that reduces the rate of protein leakage into the urine.

However, the loss of kidney function continues unless the damage caused by inflammation is controlled, leaving many treatment options ineffective.

Why DMX-200?
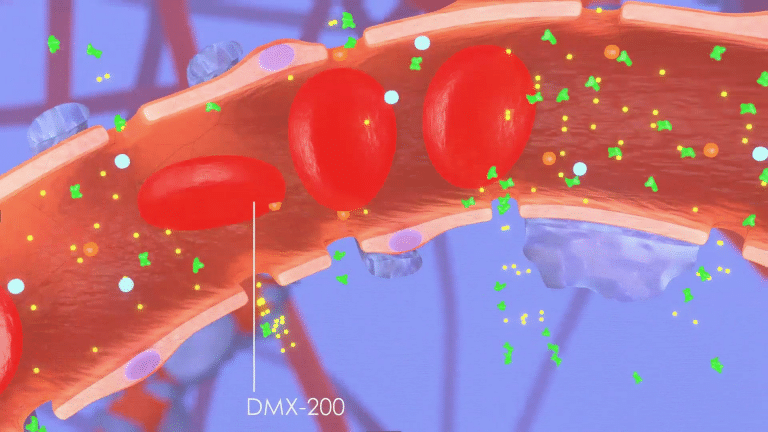
DMX-200 attacks CKD in a groundbreaking way by adding a registered anti-inflammatory drug
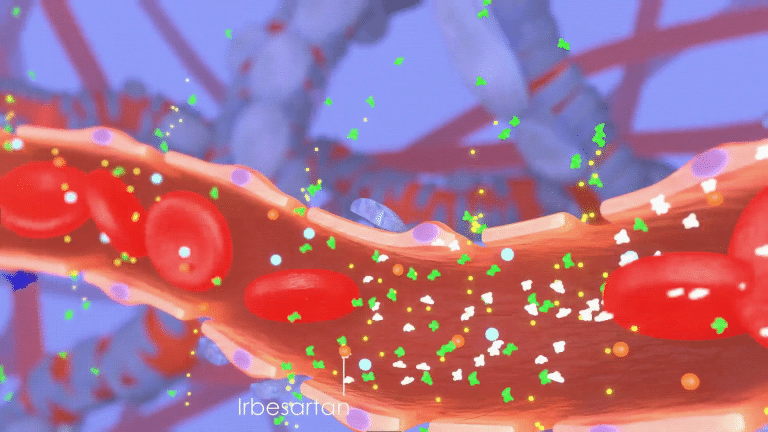
to the blood pressure lowering drug irbesartan.
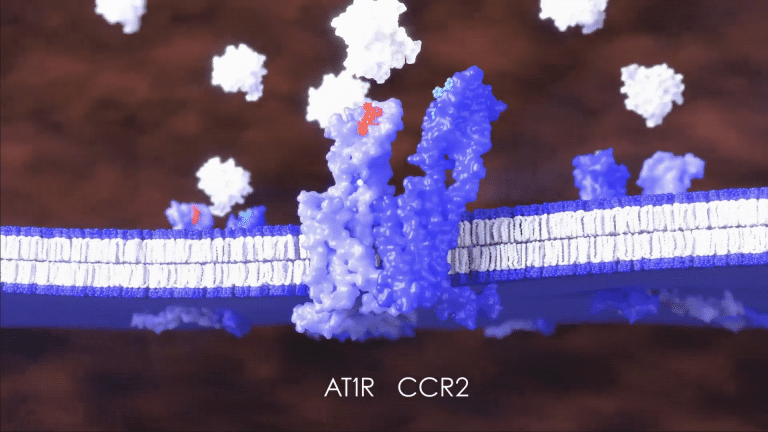
Unlike other treatments, these molecules work synergistically to block signals that attract the inflammation-causing immune cells to the kidney, limiting further damage.
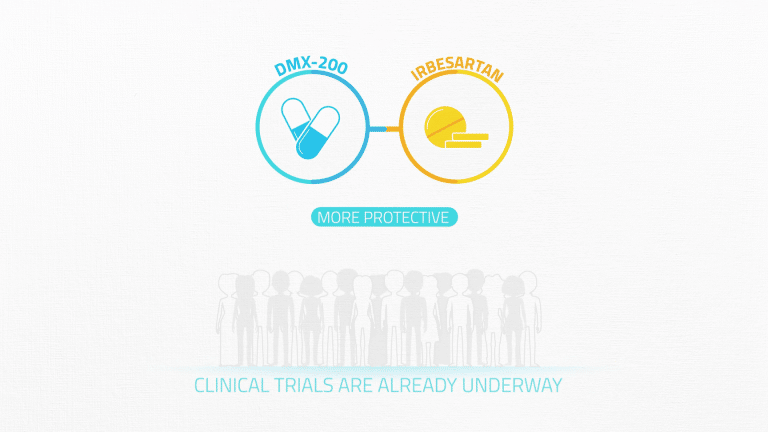
Preclinical studies have shown that together these drugs are more protective than either therapy alone, and clinical trials are already underway.

Help bring this exciting new therapeutic approach to patients with CKD —learn more about investing by emailing info@dimerix.com.